Calcite and quartz are often mistaken for each other and for good reason. These two minerals are both very abundant and have enough in common that it can be easy to mistake one for the other.
Whether you’re a budding geologist, an enthusiastic rockhound, or simply curious about the fascinating world beneath our feet, this comprehensive guide will equip you with the knowledge to distinguish between the mesmerizing mystery of calcite and the quiet elegance of quartz.
By shedding light on their subtle distinctions you’ll be able to distinguish between the two in a variety of ways including their appearance, chemical makeup, structure, and even where they are found!
Calcite Vs Quartz – The Major Differences
We’re going to start with the differences between the two. There are a lot of things in common but these are the main things you want to look out for to tell them apart (aside from the value of quartz being quite a bit higher in general) using common practices for identifying rocks and minerals:
Color – Quartz can be green and calcite can be blue

Calcite and quartz can show striking differences in color. Calcite is often seen in white or colorless shades, but it’s not limited to these.
It can actually adopt a wide array of colors, from reds to blues to greens and more. This is because of various impurities, or different substances, that mix into the calcite.
These impurities can include elements like iron or manganese, and they can dramatically change the color of the calcite.
Quartz is a bit different. In its purest form, quartz is typically colorless and clear, just like a perfect piece of glass. But, like calcite, quartz can also come in many different colors due to impurities.
Some quartz can be pink, a variety known as rose quartz, or purple, which is known as amethyst. Quartz can even be a smoky gray or brown color, or have a yellowish tint, which is known as citrine.
So, both calcite and quartz can come in many different colors, but they often start as colorless or white and get their colors from impurities in their structures.
Color isn’t the best way to tell them apart but green calcite is a color variation that is somewhat unique to calcite, largely due to the presence of certain impurities or inclusions.
Although quartz can come in a greenish color, it’s more often found in variants like prasiolite (a greenish-yellow) or aventurine (which is typically a more translucent, shimmering green due to inclusions of other minerals).
The bright, vibrant green seen in some specimens of calcite is less common in quartz.
On the other hand, blue calcite, while not entirely unique to calcite, is more common than blue quartz. Blue quartz is quite rare and usually results from inclusions or irradiation, while blue calcite often gets its color from impurities.
Luster – Calcite has a pearly look

When we talk about the luster of a mineral, we’re talking about how it reflects light, or in other words, how shiny it is. Two common minerals, calcite and quartz, can have different types of luster.
Calcite usually has a vitreous to pearly luster. ‘Vitreous’ means it shines like glass, while ‘pearly’ means it has a soft glow, like the inside of a seashell or a pearl. Depending on the specific specimen, calcite’s surface can look shiny, glossy, or even a bit waxy.
Even with its pearly luster, how much calcite is worth can be a bit surprising. Check out our guide to learn more.
Quartz, on the other hand, is known for its glassy luster. This means that it usually looks clear and shiny, just like a piece of clean, well-polished glass. This glassy look is one of the characteristics that can make quartz so appealing.
In fact, this is why quartz is often used in jewelry, as its glassy luster can make it sparkle and catch the light beautifully.
Hardness – Quartz is a lot harder

The hardness of a mineral is a measure of how easily it can be scratched. For this, geologists use the Mohs scale, which ranks minerals from 1 (softest) to 10 (hardest). On this scale, calcite scores a 3, while quartz scores a much higher 7.
Let’s talk about calcite first. Because it has a low hardness of 3, it can be scratched easily. In fact, even a copper penny, which has a hardness of just over 3, can leave a mark on calcite.
This softness is part of the reason why calcite isn’t usually used in jewelry – it could get scratched too easily!
Quartz, on the other hand, is quite hard. Its score of 7 means that it’s resistant to being scratched by most other substances. You’d need something as hard as a hardened steel file or another quartz crystal to scratch it.
This hardness is one of the reasons why quartz is often used in jewelry. It’s durable and can keep its polished, shiny surface even when worn every day.
So, when comparing calcite and quartz, remember that calcite is pretty soft and can be easily scratched, while quartz is much harder and resists scratching.
Structure – Calcite has a more blocky and chunky look

Crystal structure refers to the arrangement of atoms in a crystalline substance, and it shapes the way a crystal forms. In the case of minerals like calcite and quartz, their crystal structures differ quite a bit.
Calcite has a crystal structure that’s described as ‘trigonal’. This means that its atoms are arranged in a three-sided pattern, which usually results in calcite crystals forming shapes like a skewed cube or a crystal with faces that are all different triangles. Because of this structure, calcite often looks blocky or chunky.
Quartz, on the other hand, has a crystal structure that’s ‘hexagonal’. This means its atoms are arranged in a six-sided pattern, which often leads to quartz crystals forming long, six-sided prisms that taper to a point, or needles.
If you’ve ever seen a piece of quartz that looks like a tall, pointy tower, that’s due to its hexagonal crystal structure.
Cleavage – Quartz breaks in curved shapes

Cleavage is the way a mineral breaks along certain planes, or flat surfaces. This doesn’t mean the mineral will always break in these ways, but it’s more likely to.
For calcite and quartz, their cleavage patterns are quite different.
Calcite has what’s known as ‘perfect’ cleavage in three directions. This means it tends to break into pieces with flat, smooth surfaces. If you ever get the chance to break a piece of calcite, you’ll likely end up with fragments that have this unique, blocky shape.
Quartz, however, has no cleavage at all. When quartz breaks, it doesn’t break along flat, smooth surfaces. Instead, it breaks in a pattern known as ‘conchoidal fracture’.
This pattern results in curved, shell-like shapes that look like the inside of a clamshell or the surface of a broken piece of glass.
Calcite will glow under an ultraviolet light
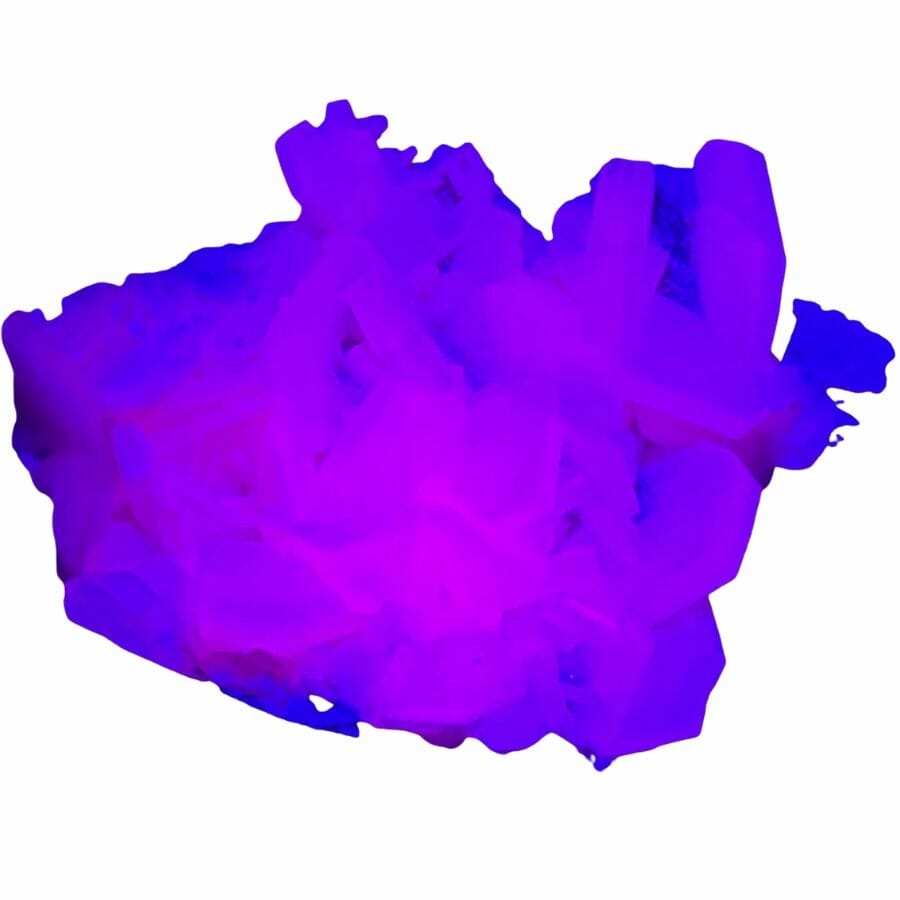
Fluorescence is a really cool property some minerals have that makes them glow under ultraviolet (UV) light. This happens because certain minerals can absorb UV light and then release it as visible light.
Calcite will often glow under UV light. This means that when you shine UV light on it, calcite can glow in various colors like red, blue, or yellow. The specific color depends on the impurities in the calcite.
For example, if it has impurities like manganese or lead, it might glow red or orange. This can make calcite really fun to look at under a UV lamp!
On the other hand, quartz typically doesn’t show fluorescence under UV light. Most of the time, if you shine UV light on quartz, it will just stay the same color.
But there are exceptions. Certain types of quartz can fluoresce, but this is usually due to specific mineral inclusions or certain types of irradiation.
Composition – Quartz is mostly silicon and oxygen

Chemical composition refers to the types of elements that make up a mineral. It’s kind of like a recipe that tells you what ingredients you need to make that mineral. The chemical compositions of calcite and quartz are different, making them distinct minerals.
Calcite is made of calcium carbonate. If we break that down, it means calcite is composed of calcium, carbon, and oxygen atoms. These elements come together to form CaCO3, the chemical formula for calcium carbonate.
This composition is quite common in the Earth and is found in many rocks, especially limestone.
Quartz, on the other hand, is made of silicon dioxide. Its chemical formula is SiO2, which means it’s composed of silicon and oxygen atoms. These two elements are also abundant in the Earth’s crust.
In fact, silicon and oxygen are the two most common elements in the Earth’s crust, and quartz is one of the most common minerals.
Formation – Calcite often forms in caves and on the ocean floor

Calcite and quartz are both common minerals, but they’re formed in different ways. These formation processes have to do with the conditions in which the minerals crystalize, or grow from a liquid or gas into a solid form.
Calcite often forms when water rich in calcium evaporates or cools down, it leaves behind calcite. This often happens in caves or on the ocean floor.
If you’ve ever seen stalactites or stalagmites in a cave, those are often made of calcite! Limestone, a type of rock often used in building, is also largely made of calcite.
Quartz forms in a different way. It usually forms as molten rock, or magma, cools down. As the magma cools, the atoms in it arrange themselves into the pattern of quartz, forming quartz crystals.
This is why quartz is common in many types of rocks, including igneous rocks (formed from cooled magma), metamorphic rocks (formed from other rocks under high heat and pressure), and sedimentary rocks (formed from bits of other rocks).
Location – Quartz is found in a lot more areas

Calcite and quartz are both found all over the world, but they tend to show up in different types of places due to their different formation processes. There are a lot of great places for rockhounding near you where you can find either of these.
Calcite is often found in sedimentary environments, where water rich in calcium carbonate evaporates or cools down. This means you can find calcite in places like caves, where it forms stalactites and stalagmites, and in areas with limestone or marble.
Calcite is also found in the shells of many marine organisms, so it’s abundant in places that were once under the sea. If you want some more examples you can take a look at our guide to gem mining near you.
Quartz, on the other hand, is one of the most common minerals in the Earth’s crust and can be found in a variety of rock types, including igneous, metamorphic, and sedimentary rocks.
You can find quartz in sand at the beach, in granite countertops, and in many mountain ranges. It’s especially common in areas with a lot of volcanic activity, where magma has cooled and crystallized into rock.
Price – Quartz tends to be a lot more valuable

The price of calcite vs quartz can vary a lot depending on a few factors. These can include the size of the specimen, its quality, and whether it has any special features, like unusual crystal forms or colors.
Both are some of the more common minerals on Earth so the average prices and values tend to be pretty low. You can find them in a lot of different places, and they often form large, clear crystals.
However, there are several varieties of quartz that are highly sought after and can form gemstone-quality specimens. These more valuable types can fetch a pretty penny. For more specific information on pricing check out our guides below on the different types of quartz:
- Pricing guide for amethyst
- Pricing guide for citrine
- Pricing guide for quartz
- Pricing guide for calcite
Quartz Vs Calcite – The Similarities
Now that we’ve gone over the main differences between quartz and calcite we’re going to cover how they are similar. These tests will typically give you similar results:
Streak – Both will usually leave a white trail

In mineralogy, the term ‘streak’ refers to the color of the powder left behind when a mineral is scratched across a rough surface like a piece of unglazed porcelain tile, known as a streak plate. Interestingly, the color of the streak can be quite different from the color of the mineral in its solid form.
Now, let’s talk about the streaks of calcite and quartz, two common minerals. Both calcite and quartz share a similar characteristic when it comes to their streak – they both typically leave a white streak.
No matter what color the mineral appears in its solid form, if you were to scratch a piece of calcite or quartz across a streak plate, the powder left behind would most likely be white.
This is one of the reasons why streak is such a useful tool for identifying minerals. Even if the mineral comes in many different colors, like calcite and quartz can, the color of its streak can help you figure out what it is.
Density – They will feel about the same weight

Density is a measure of how much matter is packed into a certain amount of space. That means that when you have two objects of the same size the denser one will feel heavier even if it takes up the same amount of space.
For minerals, density can help us understand their composition and structure. Let’s look at the densities of two minerals, calcite and quartz.
Calcite has a density of about 2.71 grams per cubic centimeter. This means if you had a cube of calcite that was one centimeter on each side, it would weigh 2.71 grams.
Quartz is a bit denser than calcite, with a density of around 2.65 grams per cubic centimeter. So, a one centimeter cube of quartz would weigh 2.65 grams.
While there’s a small difference, these densities are quite similar compared to many other minerals. For instance, gold has a density of 19.32 g/cm^3, which is much higher than either calcite or quartz.
Magnetism – Neither are magnetic

Interestingly, both calcite and quartz are similar in that they are not magnetic. This means they do not attract or repel other materials due to a magnetic field.
If you held a magnet up to a piece of calcite or quartz, you wouldn’t see any interaction or feel a pull. It’s like they’re ignoring the magnet entirely!
Why is this the case? Well, magnetism is all about the electrons in an atom and how they move. In magnetic materials, certain electrons align in a way that creates a magnetic field.
But in calcite and quartz, the electrons don’t align this way, so no magnetic field is created.
Conductivity – They are both bad conductors

Conductivity is a material’s ability to allow the flow of electricity or heat. It’s why metal pots are used for cooking – metals are good conductors of heat!
But what about minerals like calcite and quartz? How do they stack up when it comes to conductivity?
Well, both calcite and quartz are quite similar in this regard – they are poor conductors of electricity. This means if you tried to pass an electrical current through a piece of calcite or quartz, it wouldn’t go through very well.
This is because the atoms in these minerals are tightly bound together and don’t allow electrons to move freely, which is necessary for electrical conductivity.
Similarly, they are also poor conductors of heat. If you heat one end of a quartz or calcite crystal, the other end won’t immediately become hot. The heat takes time to pass through these minerals due to their dense crystal structures.
The Easiest Ways To Tell Quartz and Calcite Apart

Now that you know all of the differences and similarities of quartz and calcite we’re going to recommend a few simple ways to tell them apart. These will give you a very good idea of what you have without going through a lot of trouble:
Scratch it with a steel nail to test the hardness
One of the easiest ways to distinguish between calcite and quartz is by their hardness. Quartz is much harder than calcite.
On the Mohs hardness scale, which measures the ability of a mineral to resist scratching, quartz rates a 7, while calcite rates only a 3. This means that quartz can scratch calcite, but calcite can’t scratch quartz.
You can perform a scratch test using a common steel nail, which has a hardness of about 4.5. The nail will scratch calcite but not quartz.
Look for smooth or curved breaks in the crystal
Another way to tell the two apart is by looking at their cleavage, which is the way they break. Calcite has perfect cleavage in three directions, creating rhombus-shaped fragments when broken.
Quartz, on the other hand, has no cleavage. It breaks in a pattern called conchoidal fracture, which produces curved surfaces, similar to what you see when glass breaks.
Put a little vinegar on it and look for bubbling
The way calcite and quartz interact with acid is another telltale sign. Calcite reacts with weak acids, such as vinegar, and produces bubbles of carbon dioxide.
Quartz does not react to acids. So, if you place a few drops of vinegar on the mineral and see fizzing or bubbling, it’s probably calcite, not quartz.

